Glial cells, often overshadowed by neurons, are indispensable components of the central nervous system (CNS), outnumbering neurons and providing critical support for neural function, maintenance, and protection. This diagram illustrates the four main types of glial cells in the CNS—astrocytes, oligodendrocytes, microglia, and ependymal cells—depicted in their typical interactions with neurons, highlighting how they insulate axons, regulate the extracellular environment, defend against pathogens, and facilitate fluid movement. Understanding these cells reveals their dynamic roles beyond mere support, including active participation in synaptic signaling and response to injury, essential for overall brain health and spinal cord integrity.
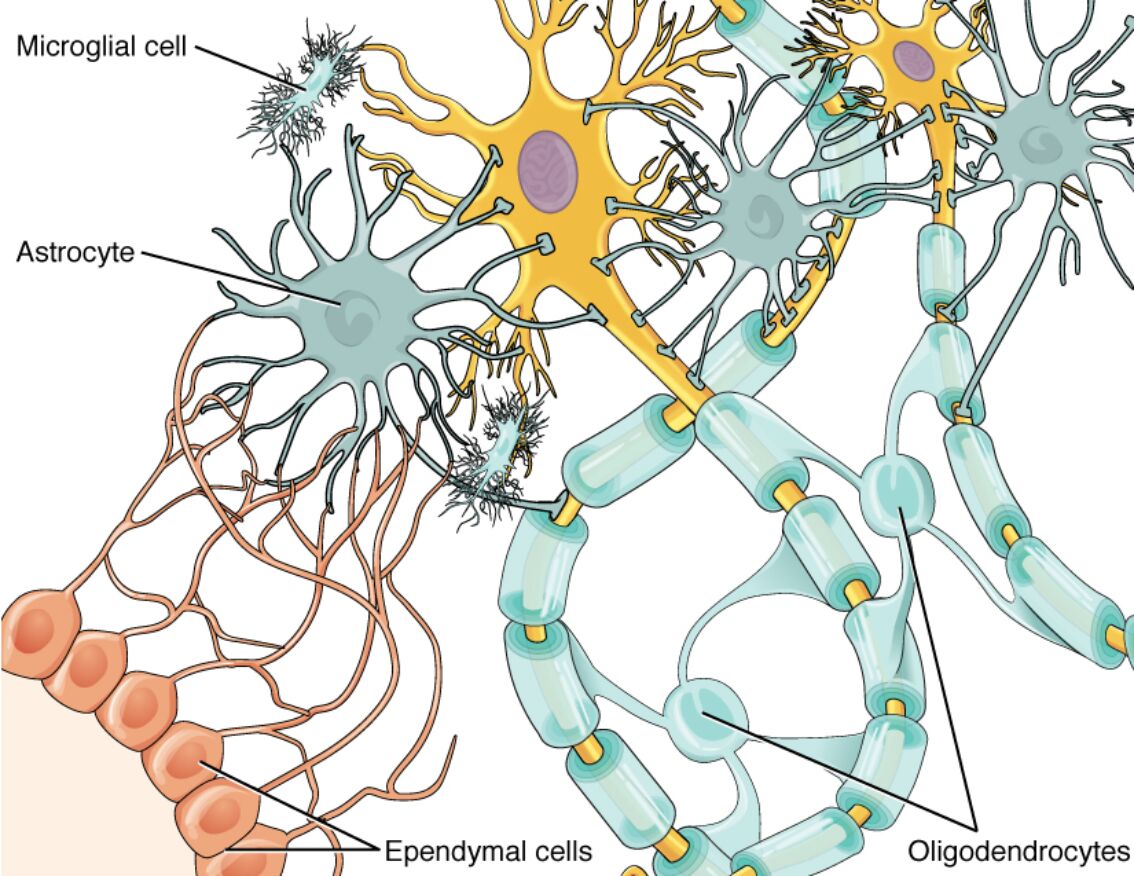
Labeled Glial Cells
Microglial cell
The microglial cell is depicted with a small, elongated body and highly branched processes that constantly survey the CNS microenvironment for damage or infection. As the resident immune cells of the brain, microglia activate to phagocytose debris, release cytokines, and promote inflammation or repair, playing a pivotal role in neuroinflammation and neurodegenerative processes.
Astrocyte
Astrocytes appear as star-shaped cells with numerous radiating processes that contact blood vessels, neurons, and synapses, forming a supportive network. They maintain ion homeostasis by uptaking excess potassium and glutamate, contribute to the blood-brain barrier via endfeet on capillaries, and supply neurons with nutrients like lactate through the astrocyte-neuron lactate shuttle.
Ependymal cells
Ependymal cells are shown as cuboidal or columnar epithelial cells lining the ventricles and central canal, often with cilia or microvilli on their apical surfaces. These cells produce and circulate cerebrospinal fluid (CSF) through ciliary beating, act as a barrier between CSF and neural tissue, and in some regions, serve as neural stem cells capable of generating new neurons or glia post-injury.
Oligodendrocytes
Oligodendrocytes are illustrated with multiple processes extending to wrap around neuronal axons, forming segmented myelin sheaths. Each oligodendrocyte can myelinate up to 50 axonal segments in the CNS, enhancing action potential conduction via saltatory propagation and providing metabolic support to axons through monocarboxylate transporters.
In-Depth Anatomy of CNS Glial Cells
The morphology of glial cells is finely tuned to their specialized environments within the brain and spinal cord. Anatomical features enable intimate associations with neurons and vasculature.
- Astrocytes exhibit two main subtypes: protoplasmic in gray matter with bushy processes and fibrous in white matter with straighter extensions, both rich in intermediate filaments like GFAP for structural resilience.
- Oligodendrocytes have a small soma with few organelles, but their processes contain myelin basic protein and proteolipid protein essential for compact myelin layers.
- Microglia in resting state display ramified branches covering territories up to 50 micrometers, transforming into amoeboid forms during activation with retracted processes.
- Ependymal cells form tight junctions creating a selective barrier, with basal processes sometimes extending into periventricular regions for additional support.
- Overall, glial cells comprise about 50% of CNS volume, with ratios varying by region—higher in cortex for astrocytes, denser in white matter for oligodendrocytes.
Physiological Functions of Glial Cells
Glial cells actively participate in neural signaling and homeostasis, far from passive roles. Their functions integrate metabolic, protective, and modulatory activities.
- Astrocytes regulate synaptic transmission by ensheathing synapses and expressing transporters like EAAT1/2 for glutamate clearance, preventing excitotoxicity.
- They also release gliotransmitters such as ATP and D-serine via calcium-dependent mechanisms, influencing neuronal excitability and plasticity.
- Oligodendrocytes not only insulate but also transfer energy substrates like pyruvate to axons, supporting high-energy demands during rapid firing.
- Microglia sense ATP gradients from damaged cells through P2Y receptors, migrating to sites for pruning unnecessary synapses during development via complement tagging.
- Ependymal cells facilitate CSF flow at rates of 0.3-0.5 ml/min in humans, with motile cilia generating directional currents essential for nutrient distribution and waste removal.
- Collectively, these cells form tripartite synapses with neurons, where astrocytic processes modulate information flow.
Glial Cells in CNS Homeostasis and Repair
Maintenance of the CNS environment relies on glial orchestration of barriers and responses. Repair mechanisms highlight their regenerative potential.
- The blood-brain barrier, fortified by astrocytic endfeet expressing aquaporin-4 channels, controls molecular passage and water balance to prevent edema.
- Oligodendrocyte precursor cells (OPCs) persist in adulthood, differentiating into mature forms to remyelinate demyelinated axons in response to signals like PDGF.
- Microglia transition phenotypes from pro-inflammatory M1 (releasing IL-1β, TNF-α) to anti-inflammatory M2 (secreting IL-10, TGF-β) for tissue resolution post-injury.
- Ependymal cells in the subventricular zone harbor stem cell niches, proliferating under FGF-2 stimulation to generate olfactory bulb interneurons.
- Glial scar formation by reactive astrocytes post-trauma limits damage spread but can inhibit axonal regrowth via chondroitin sulfate proteoglycans.
Interactions Between Glial Cells and Neurons
Glial-neuronal crosstalk is bidirectional and essential for circuit function. These interactions influence development, activity, and pathology.
- Astrocytes synchronize with neuronal calcium waves, propagating signals across gap junctions formed by connexin-43 for network-wide regulation.
- Oligodendrocytes adjust myelin thickness based on axonal diameter and activity, optimizing conduction via activity-dependent myelination cues like neuregulin-1.
- Microglia contact synapses with processes, stripping them during sleep or stress to refine connectivity, guided by fractalkine signaling from neurons.
- Ependymal cells interact with subependymal neurons, providing a niche for neurogenesis through secreted factors like noggin that antagonize BMP signaling.
- In ensembles, glia buffer extracellular pH and ions during high neuronal activity, preventing acidosis that could impair synaptic vesicle release.
Advances in Glial Cell Research
Emerging studies redefine glia as active players in cognition and disease. Techniques uncover molecular intricacies.
- Single-cell transcriptomics reveals astrocyte heterogeneity, with subtypes expressing unique genes like Aldh1l1 for metabolic specialization.
- Optogenetic manipulation of microglia via channelrhodopsin allows precise control of inflammatory responses in vivo models.
- High-resolution imaging like electron tomography visualizes oligodendrocyte myelin wrapping, showing cytoplasmic channels for nutrient exchange.
- CRISPR editing in ependymal cells explores ciliopathy genes, linking mutations to hydrocephalus phenotypes.
- Functional MRI correlates glial activation with bold signals, suggesting their contribution to hemodynamic responses traditionally attributed to neurons.
Potential Pathologies Involving Glial Cells
Though the image depicts healthy structures, glial dysfunction underlies many CNS disorders. Pathological insights guide therapeutic targets.
- Astrocytomas arise from malignant astrocytes, graded by WHO criteria, with IDH mutations affecting metabolism and prognosis.
- Oligodendrocyte loss in multiple sclerosis leads to demyelination, with autoantibodies targeting MOG protein exacerbating plaques.
- Microglial overactivation in Alzheimer’s promotes amyloid-beta aggregation via NLRP3 inflammasome, accelerating plaque formation.
- Ependymal cell defects in ciliopathies like Joubert syndrome impair CSF flow, causing ventriculomegaly and developmental delays.
- Gliosis in epilepsy involves hypertrophic astrocytes altering potassium buffering, lowering seizure thresholds through reduced Kir4.1 channels.
In conclusion, the glial cells illustrated—astrocytes, oligodendrocytes, microglia, and ependymal cells—form a sophisticated support system integral to CNS operation, from insulation and immunity to fluid dynamics and repair. Recognizing their multifaceted contributions not only deepens appreciation of neural complexity but also opens avenues for innovative treatments in neurological conditions, fostering ongoing advancements in neuroscience.
